Text Version
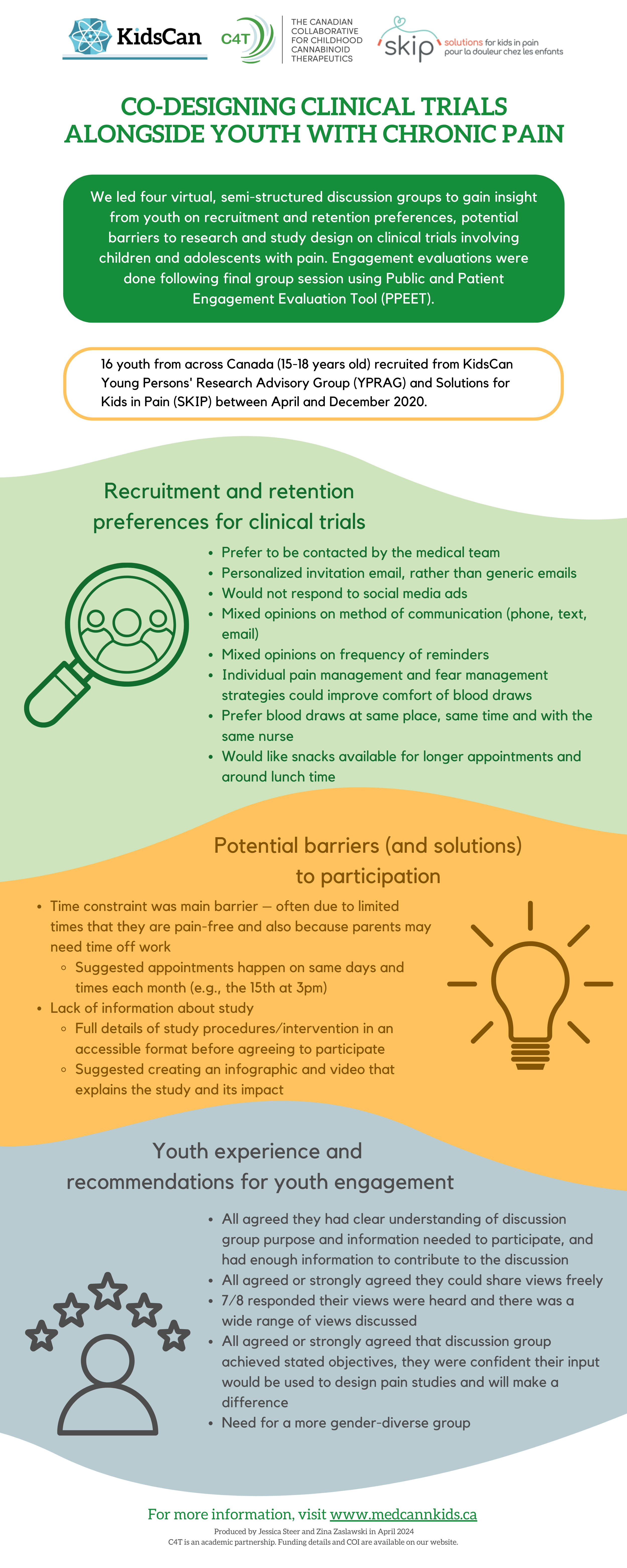
CO-DESIGNING CLINICAL TRIALS ALONGSIDE YOUTH WITH CHRONIC PAIN
We led four virtual, semi-structured discussion groups to gain insight from youth on recruitment and retention preferences, potential barriers to research and study design on clinical trials involving children and adolescents with pain. Engagement evaluations were done following final group session using Public and Patient Engagement Evaluation Tool (PPEET).
16 youth from across Canada (15-18 years old) recruited from KidsCan Young Persons' Research Advisory Group (YPRAG) and Solutions for Kids in Pain (SKIP) between April and December 2020.
Recruitment and retention preferences for clinical trials
Prefer to be contacted by the medical team
Personalized invitation email, rather than generic emails
Would not respond to social media ads
Mixed opinions on method of communication (phone, text, email)
Mixed opinions on frequency of reminders
Individual pain management and fear management strategies could improve comfort of blood draws
Prefer blood draws at same place, same time and with the same nurse
Would like snacks available for longer appointments and around lunch time
Potential barriers (and solutions) to participation
Time constraint was main barrier – often due to limited times that they are pain-free and also because parents may need time off work
Suggested appointments happen on same days and times each month (e.g., the 15th at 3pm)
Lack of information about study
Full details of study procedures/intervention in an accessible format before agreeing to participate Suggested creating an infographic and video that explains the study and its impact
Youth experience and recommendations for youth engagement
All agreed they had clear understanding of discussion group purpose and information needed to participate, and had enough information to contribute to the discussion
All agreed or strongly agreed they could share views freely
7/8 responded their views were heard and there was a wide range of views discussed
All agreed or strongly agreed that discussion group achieved stated objectives, they were confident their input would be used to design pain studies and will make a difference
Need for a more gender-diverse group
For more information, visit www.medcannkids.ca
Produced by Jessica Steer and Zina Zaslawski in April 2024.
C4T is an academic partnership. Funding details and COI are available on our website.