Text Version
CLINICIAN VIEWS ON AND ETHICS PRIORITIES FOR AUTHORIZING MEDICAL CANNABIS IN THE CARE OF CHILDREN AND YOUTH IN CANADA: A QUALITATIVE STUDY
Semi-structured interviews with clinicians involved in pediatric care across Canada explored how ethical priorities (e.g., access, safety, autonomy) effect clinicians' decisions for authorizing use of medical cannabis among children and youth.
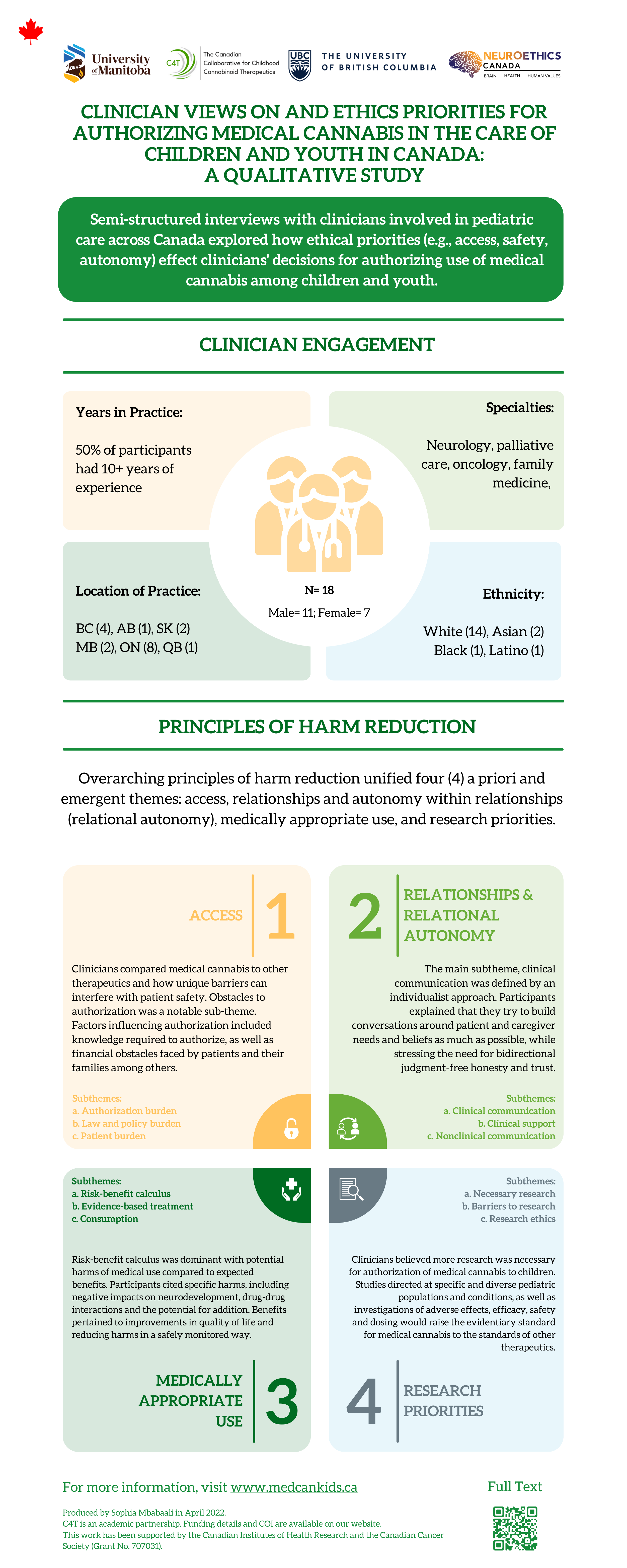
Clinician Engagement
N=18; Male=11, Female=7
Years in Practice: 50% of participants had 10+ years of experience.
Specialties: Neurology, palliative care, oncology, family medicine.
Location of Practice: BC (4), AB (1), SK (2), MB (2), ON (8), QB (1)
Ethnicity: White (14), Asian (2), Black (1), Latino (1)
Principles of Harm Reduction
Overarching principles of harm reduction unified four (4) a priori and emergent themes: access, relationships and autonomy within relationships (relational autonomy), medically appropriate use, and research priorities.
Access
Clinicians compared medical cannabis to other therapeutics and how unique barriers can interfere with patient safety. Obstacles to authorization was a notable sub-theme. Factors influencing authorization included knowledge required to authorize, as well as financial obstacles faced by patients and their families among others.
Subthemes:
Authorization burden
Law and policy burden
Patient burden
Relationships & Relational Autonomy
The main subtheme, clinical communication was defined by an individualist approach. Participants explained that they try to build conversations around patient and caregiver needs and beliefs as much as possible, while stressing the need for bidirectional judgment-free honesty and trust.
Subthemes:
Clinical communication
Clinical support
Nonclinical communication
Medically Appropriate Use
Risk-benefit calculus was dominant with potential harms of medical use compared to expected benefits. Participants cited specific harms, including negative impacts on neurodevelopment, drug-drug interactions and the potential for addition. Benefits pertained to improvements in quality of life and reducing harms in a safely monitored way.
Subthemes:
Risk-benefit calculus
Evidence-based treatment
Consumption
Research Priorities
Clinicians believed more research was necessary for authorization of medical cannabis to children. Studies directed at specific and diverse pediatric populations and conditions, as well as investigations of adverse effects, efficacy, safety and dosing would raise the evidentiary standard for medical cannabis to the standards of other therapeutics.
Subthemes:
Necessary research
Barriers to research
Research ethics
For more information, visit www.medcankids.ca
Produced by Sophia Mbabaali in April 2022.
C4T is an academic partnership. Funding details and COI are available on our website.
This work has been supported by the Canadian Institutes of Health Research and the Canadian Cancer Society (Grant No. 707031).